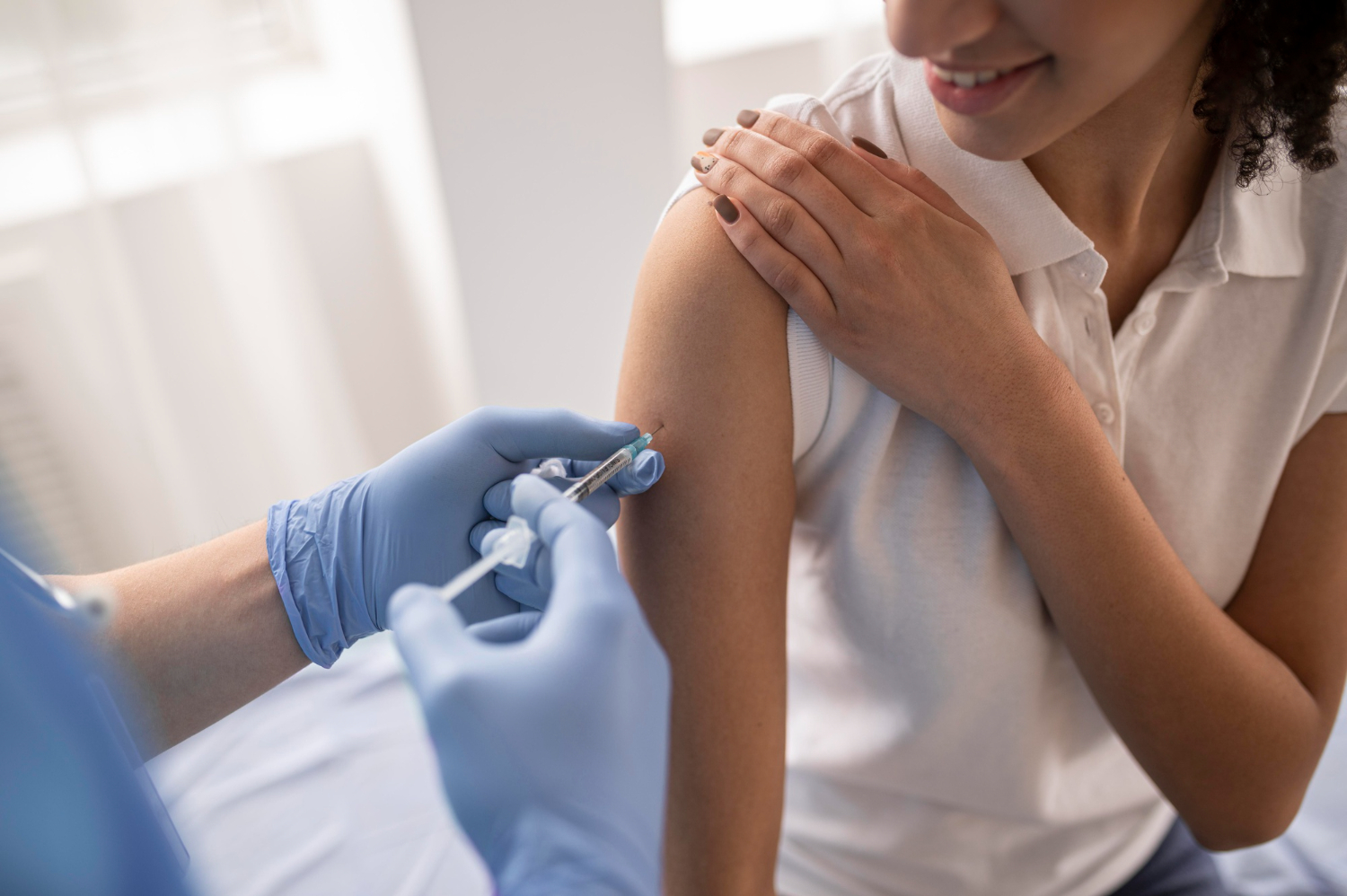
Surrey medics, in collaboration with doctors in Hampshire and Australia, have launched a groundbreaking clinical trial for a vaccine aimed at treating early-stage bowel cancer. The trial, led by Dr Tony Dhillon, a consultant oncologist at the Royal Surrey NHS Foundation Trust, has the potential to provide real hope for patients in their fight against this devastating disease. With plans to enroll 44 patients over an 18-month period, the trial is expected to make significant progress in the quest to eradicate bowel cancer.
1. Collaborative Effort to Eradicate Bowel Cancer
The groundbreaking bowel cancer vaccine trial is a collaborative effort between doctors in Surrey, Hampshire, and Australia. The Cancer Research UK Southampton clinical trials unit at the University of Southampton, alongside the Royal Surrey NHS Foundation Trust and the Queen Elizabeth Hospital in Adelaide, will be running the trial. This multinational collaboration aims to combine expertise and resources to accelerate progress in the fight against the third most common cancer type.
2. Hope for Patients in the Fight Against the Disease
Dr Tony Dhillon, the chief investigator of the trial, expressed high hopes for the success of the vaccine. After four years of development in collaboration with Prof Tim Rice in Australia, Dr Dhillon believes that the vaccine has the potential to completely eradicate the cancer in many patients. This breakthrough brings real hope to individuals battling bowel cancer and provides an opportunity for them to overcome the disease.
3. The Trial: How It Works
A total of 44 patients will be enrolled in the trial over the course of 18 months. The first patient is expected to be enrolled within the next two months. Eligibility for the trial involves an endoscopy and a tissue sample test. If deemed suitable, patients will receive three doses of the vaccine before undergoing surgery to remove the cancer. If successful, the vaccine could be licensed for use in two years, with further studies conducted for patients with later stage bowel cancer.
4. Roadmap for the Future
The current trial represents a significant milestone in the fight against bowel cancer. If successful, this vaccine could revolutionize treatment options and outcomes for patients. Further research and studies will be conducted to explore the vaccine’s efficacy for individuals with more advanced stages of the disease. The collaboration between the UK and Australia highlights the importance of international partnerships in advancing cancer research.
5. Trust’s CEO Emphasizes the Importance of the Vaccine Trial
Louise Stead, the chief executive of the Royal Surrey NHS Foundation Trust, emphasized the significance of the vaccine trial for bowel cancer patients. She stated that the trial provides real hope for patients, giving them an opportunity to overcome the disease. The potential licensing of the vaccine within two years is a promising prospect that could change the landscape of bowel cancer treatment.
FAQs:
- What is the goal of the bowel cancer vaccine trial?
The goal of the trial is to develop a groundbreaking vaccine for the treatment of early-stage bowel cancer. - How many patients will be enrolled in the trial?
A total of 44 patients will be enrolled in the trial over an 18-month period. - How does the vaccine trial work?
Eligible patients will receive three doses of the vaccine before undergoing surgery to remove the cancer. - When could the vaccine be licensed for use?
If successful, the vaccine could be licensed for use within two years.
Will the vaccine be effective for individuals with advanced-stage bowel cancer? Further studies will be conducted to explore the efficacy of the vaccine for individuals with later stage bowel cancer.